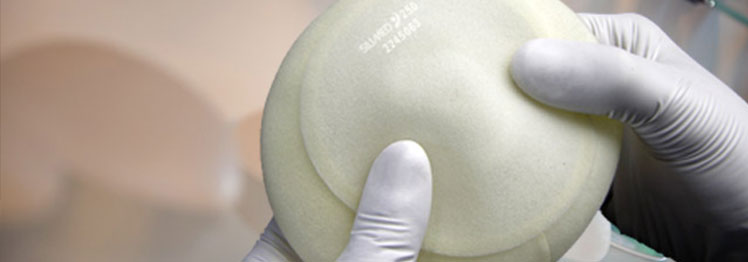
The implant name ‘Furry Brazilian’ is a fancy nick-name for a polyurethane foam coated implant manufactured by a Brazilian Company. The implant is made of silicone gel just like a typical silicone implant but with an outer shell made from polyurethane foam. It was introduced in the early 1970s and you won’t be surprised to hear that they have a furry texture and suede-like appearance. They stopped being used in the United States in 1992 when all silicone implants were banned, and despite silicone gel implants now being available again in the US, Brazilian Furry implants are still not approved by the FDA – the US Food and Drug Administration. They are also not approved for use in Thailand by the regulatory health authorities.
Why do some Surgeons in other countries still use these?
There have been some studies which have documented that by using the furry implants there is a reduced risk of Capsular Contracture – a side-effect of some Breast Augmentation procedures, where the body reacts to the implant by forming a protective lining around it and hardening. However, Capsular Contracture is something that is not covered under standard surgical guarantees in any country, as it can occur as a result of a number of issues not directly related to the actual surgery or implant itself, but instead the patient’s own response to a foreign object.
Why our CosMediTour Plastic Surgeons prefer not to offer these
Most of our Plastic Surgeons recommend against the use of Furry Brazilian Implants for our clients for one or both of these reasons:
1. Future Revision Surgery
No implants last forever, and if it isn’t the actual implant that requires replacing in future, the patient may choose revision because of changes to the aesthetic appearance of her breasts. In this case, removal of Brazilian Furry or Fuzzy Implants requires the removal of the patient’s breast pocket tissue that has bonded to the polyurethane foam coating of the implants. A lot more of the patient’s tissue is removed, whereas with traditional smooth or textured implants, the tissue has not bonded to the implant surface, making implant removal and revision much less traumatic on the patient’s body.
2. Potential Carcinogenic Issues
There are still questions as to whether these implants represent an increased Carcinogenic risk due to the polyurethane foam coating, which breaks down in the patient’s body over time. It is for this reason they are not yet approved by the FDA (Federal Drug Administration) in the United States of America. Where this polyurethane goes is still a mystery, because when these implants are removed after a number of years, some of the polyurethane foam coating has disappeared. It still remains unclear what the body does with these materials, and many researchers have concerns that polyurethane can lead to chronic inflammation and cancer.
Textured or Smooth Implants?
Most surgeons prefer to use textured silicone gel implants as this helps minimise Capsular Contracture and movement of the implant where it is not aesthetically pleasing.
You will have the opportunity to discuss these options with your surgeon during your face-to-face consultation as to what type of implant will give you the best outcome being Smooth or Textured, based on your desires and your own anatomy.
Further Reading
For more information on Furry or Fuzzy Brazilian Implants see these articles and blogs:
http://www.cosmeticsurgeryoz.com/Brazilian-breast-implants.htm
http://www.realself.com/question/opinions-and-furry-brazilian-implants