Interested in the safety of Breast Implant microchips? We get it – safety is crucial when considering Breast Augmentation or Breast Augmentation with Lift. In our latest blog, we’ll explore Motiva Qid® Technology, breaking down its functionality, purpose, and, of course, addressing the key question: Are Breast Implant Microchips safe?
INDEX
WHAT IS MOTIVA QID® TECHNOLOGY?
Motiva Qid® Technology, also known as Q Inside Safety technology, is incorporated into Motiva Breast Implants to enhance patient safety.
Qid® functions as a microtransponder or ‘microchip’ embedded within Motiva implants, storing comprehensive details about the Breast Implant, such as:
- Breast Implant Brand
- Implant Type
- Breast Implant Size (CCs)
- Breast Implant Serial Number
- Surgery Location
- Manufacturing Information
- Surgery Date
In the unlikely event of an implant recall or issues with your Breast Implants, this digital record-keeping system proves invaluable. It provides a swift and efficient means of identification without requiring you to locate and present physical paperwork.
HOW DOES MOTIVA QID® WORK?
Utilising a 12mm micro transponder, Motiva Qid® can be scanned externally with a handheld reader. This scanning process reveals the implant’s electronic serial number (ESN), serving as the key to access all the detailed information about the implant.
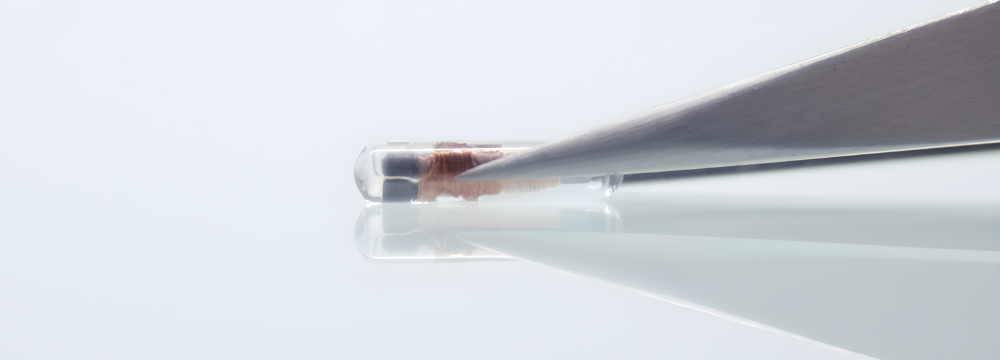
ARE BREAST IMPLANT MICROCHIPS SAFE?
Extensive testing is conducted on all Breast Implants before they reach the market. The safety of Qid® technology is validated by:
- The Therapeutic Goods Administration in Australia
- The European Economic Area, where Qid® carries the CE mark on the micro transponder within Motiva implants. This mark signifies rigorous testing and adherence to health, safety, and environmental standards.
- The United States Food & Drug Administration (FDA), which cleared it for use in 2004.
While these microchips bring advanced safety measures, it’s crucial to have open discussions with your Plastic Surgeon to address any concerns. Whether you’re considering Breast Augmentation or have specific worries about a particular type of breast implant, raising these concerns during your consultation is essential.
Always ensure you undergo a thorough consultation with a qualified Plastic Surgeon before making any decisions about any procedure.
BENEFITS OF BREAST IMPLANT MICROCHIPS
Motiva Qid® technology offers a range of advantages for those considering breast implants, including:
IMMEDIATE IDENTIFICATION
The microchip facilitates efficient digital record-keeping of crucial details such as brand, size, serial number, volume, and surgery information.
LONG-TERM MONITORING
Enables continuous monitoring of implant information, ensuring ongoing patient safety and well-being.
AIDS REVISION SURGERY
Simplifies the planning for Breast Revision Surgery by providing easily accessible details about your current implants.
IMPLANT RECALL
In the rare event of an implant recall, the micro transponder can be scanned, streamlining the identification process.
IS THE QID® MICROCHIP IN MY BREAST IMPLANT SAFE FOR MRI?
Further Information
To learn more about Breast Implants or Breast Augmentaiton surgery, please connect with our friendly Client Support Team.